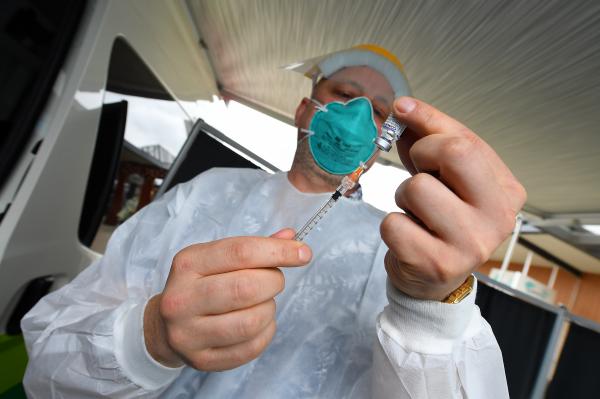
A collaborative effort between Victorian scientists, researchers and manufacturers has produced Australia’s first mRNA vaccine candidate.
450 doses have been produced in Boronia, enabling 150 people to take part in phase 1 clinical trials.
The project has been led by mRNA Victoria in partnership with Monash Institute of Pharmaceutical Sciences, the Doherty Institute and IDT Australia.
Results from the trials being run by the Doherty Institute are expected in 2022.
Doherty Institute Head of Vaccine and Immunisation research group Professor Terry Nolan said this is a major milestone in Australia’s ability to manufacture home-grown Covid-19 mRNA vaccines.
“We are excited to commence Phase 1 clinical trials of this candidate, along with the protein vaccine candidate developed by the Doherty Institute, in the coming months.” He said.
The Victorian state government invested $5 million in equipment to enable IDT Australia to manufacture the vaccine candidate.
Minister for Innovation, Medical Research and the Digital Economy Jaala Pulford said the vaccine candidate was an Australian first.
it is an incredible achievement to have made an mRNA vaccine candidate that is ready for clinical trials.” She said.
“We’re serious about developing our mRNA manufacturing capacity and doing it quickly as we can, because it will save lives.”
The vaccine will be required to pass three stages of clinical trials before being subject to approval by the Therapeutic Goods Administration.
Monash University Professor of Pharmaceutical Biology Colin Pouton said he has worked closely with IDT to develop the vaccine candidate.
“Reaching this milestone demonstrates that the skills and experience to make mRNA products are available in Victoria.” He said.
The vaccine candidate has been developed over the past five months before reaching the clinical trial stage.
IDT Australia chief executive officer Dr David Sparling said the company is honoured to be a part of the collaborative effort.
“We believe this product will be the first locally developed mRNA COVID-19 vaccine candidate and the first locally manufactured cGMP mRNA drug product.”